EUROArray Dermatomycosis
The test system for doctor's practices and laboratories
The EUROArray Dermatomycosis is a PCR-based procedure for detection of 56 fungi species causing infections of skin, hair and nails. Out of these 56 pathogens, 23 dermatophyte, 3 yeasts and 3 mould species can be identified. In the following, the procedure is explained in detail.
Sample collection
The following sample materials can be investigated with the EUROArray Dermatomycosis:

It is important to ensure that instruments used for collecting samples are not contaminated with fungal components. Before each sample collection, the area suspected of mycosis should be disinfected with 70% alcohol in order to reduce non-pathogenic germs of the transient secondary flora.
Since fungal detection by means of PCR-based tests does not require vital pathogens, dead cells can also be used. These are present in crusts or larger skin scales used in the PCR-based EUROArray Dermatomycosis.
To obtain a short guide on collecting samples of different materials, please contact us via mdx-pm@euroimmun.de.
DNA isolation
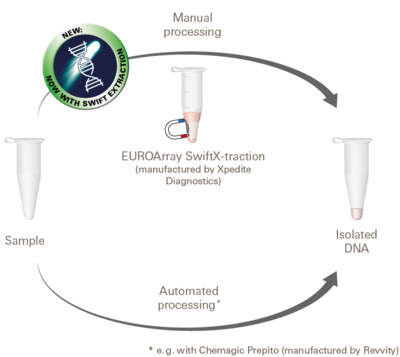
For analysis using the EUROArray Dermatomycosis, the pathogen DNA required for the detection must be isolated from the sample material. The necessary amount and quality of DNA is generally obtained using common products for DNA isolation, especially with EUROArray SwiftX-traction. The underlying method is particularly fast and easy to use. With larger sample numbers, the DNA isolation can also be performed automatically.
For more information on the EUROArray SwiftX-traction kit, visit the website of Xpedite Diagnostics.
The instructions for use of the EUROArray SwiftX-traction kit are available for download here.
Should you require more information or have any questions, do not hesitate to contact us.
Polymerase chain reaction (PCR) and hybridisation
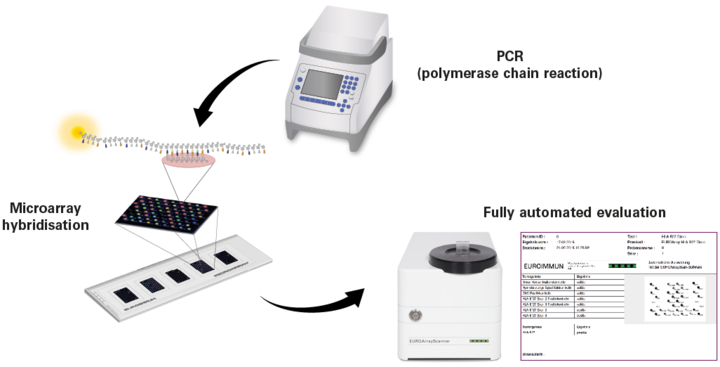
In the PCR, specific sequences of the different pathogens, as far as present in the sample, are multiplied millionfold and labelled with a fluorescent dye. During hybridisation, the PCR products then bind to specific probes at special positions on the EUROArray BIOCHIPs. Based on the fluorescence signals, the PCR products can be made visible and, depending on the location of the PCR products, clearly allocated to the respective pathogens.
All required reagents are included in EUROArray kit. For the PCR, the ready-to-use PCR reagents (Mix A and B) are just mixed together, and the DNA isolated from the patient sample is added to the solution. The amplification of the DNA is performed in the Thermocycler according to the protocol included in the test instruction.
For the hybridisation, the PCR product is mixed with a hybridsation buffer and then incubated with EUROArray slides, which contain three or five BIOCHIPs depending on the format. One field is used per patient. This step is performed on a hybridisation station provided by EUROIMMUN, at a temperature of 55°C.
Numerous integrated controls ensure that sufficient DNA for each sample has been added to the PCR, that both PCR and hybrisiation have worked correctly, and that no cross reactions have occurred between adjacent samples during hybridisation.
To obtain a short guide on the required work steps, please contact us via mdx-pm@euroimmun.de.
Fully automated evaluation using the EUROArrayScanner

The combination of the EUROArrayScanner and EUROArrayScan software allows easy, quick and objective evaluation of the EUROArrays without the necessity of studying complicated manuals. With this evaluation unit from EUROIMMUN, all results of patient samples analysed in parallel can be evaluated and archived in the shortest time. Moreover, the software allows fast and simple generation of report printouts containing the respective test result for each patient.
Report printout generated with the EUROArrayScan software
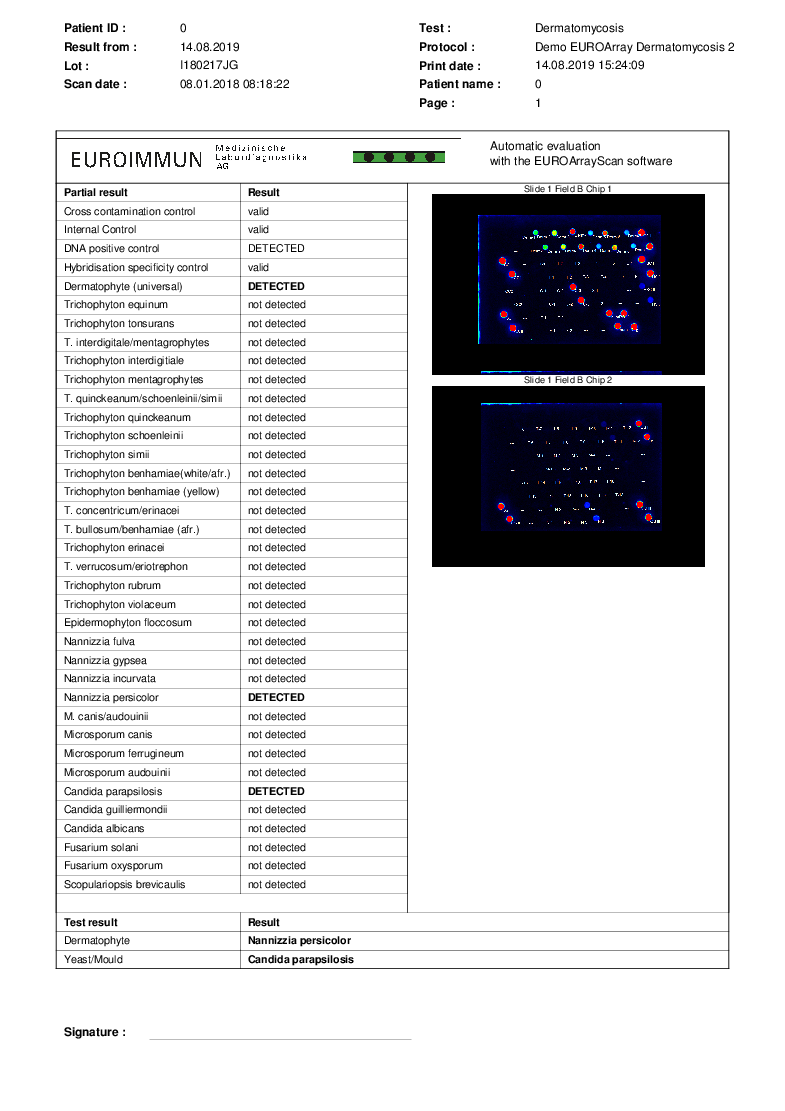
In the report printout, all results from the analysis with the EUROArray Dermatomycosis for the respective patient sample are shown in a clear overview. In the upper part of the table, the results for the integrated controls (cross contamination control, internal control, DNA positive control, hybridisation specificity control) are shown. These ensure for every single analysis that the individual procedural steps have been performed correctly and that the analysis was successful.
Below the controls, the test results for the different pathogens that are detectable with the test system are shown. Under "Test result", the final results of the analysis for the categories "Dermatophyte" and "Yeast/mould" are shown.
